Techspert, the AI technology innovator connecting businesses with experts for domain-specific...
CAR-T cell therapy is a cutting-edge immunotherapy poised to transform cancer treatment. Although there’s a lot of excitement and hope around it, there are still many unknowns. To get a better understanding of the current and future CAR-T landscape, we speak with a Professor of Medicine who is the head of a pioneering CAR-T programme at a university hospital with extensive experience in treating a range of cancers, including diffuse large B-cell lymphoma (DLBCL), multiple myeloma (MM) and acute lymphoblastic leukaemia (ALL).
Before we dig into the discussion, let’s briefly introduce chimeric antigen receptor (CAR)-T cell therapy.
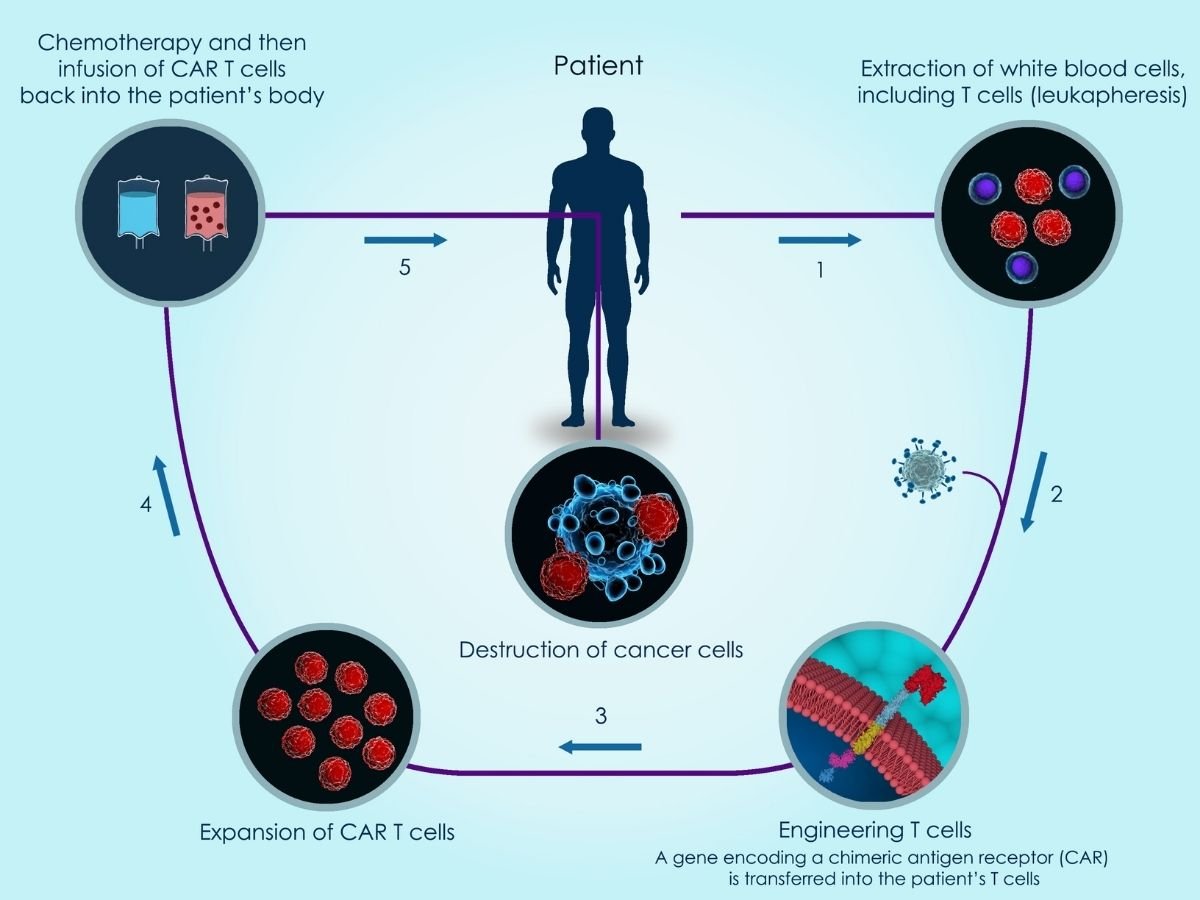
An illustration of how CAR-T cell therapy works.
CAR-T cell therapy is customized and manufactured for each individual patient. It works by taking T cells (a type of immune system cell) from a patient’s blood, inserting the CAR gene (which codes for a special receptor that binds to a specific protein on the patient’s cancer cells) into the T cells to make CAR-T cells, growing large numbers of these new chimeric antigen receptor-expressing T cells in the laboratory. These CAR-T cells are then infused back into the patient, now recognizing and attacking cancer cells. One of the extraordinary things about this treatment is that it’s a “living therapy”. Because CAR-T cells go on to multiply in the body and continue fighting cancer in the patient, they typically only have to be injected once.
With the foundation laid, it’s time to dive into our Q&A.
What is your perception of the current CAR-T cell therapy treatment pathways?
CAR-T cells that target CD19 (a surface biomarker for B lymphocytes) have become standard treatments of relapsed or refractory haematological malignancies, such as B cell acute lymphoblastic leukaemia (ALL) and relapsed or refractory large B cell lymphoma (LBCL).
In addition, several clinical trials in further tumor entities are underway, such as:
- CAR-T Hepatic Artery Infusions or Pancreatic Venous Infusions for CEA-Expressing Liver Metastases or Pancreas Cancer (HITM-SURE)
- A Study of CCT301-59 CAR T Therapy in Adult Subjects With Recurrent or Refractory Solid Tumors (CAR)
- Safety and Efficacy of CCT301 CAR-T in Adult Subjects With Recurrent or Refractory Stage IV Renal Cell Carcinoma
- CART-PSMA-TGFβRDN Cells for Castrate-Resistant Prostate Cancer
This activity makes it likely that in the near future there will be a broader clinical use of CAR-T cells with different antigen specificities.
What are the concerns, limitations and benefits of CAR-T therapy?
The safety of CAR-T cells is of major concern since this new class of anti-tumor therapy has previously unknown side effects. The complete risk profile of CD19+ CAR-T cells could not been defined in the relatively small initial trials leading to approval of CAR-T cell products. This led to the obligation to document toxicities in any patient receiving commercial CAR-T cell products. Currently, there is emerging evidence demonstrating both short-term and medium-term effects which were in part unknown at the time of regulatory approval.
A major limitation is our incomplete understanding on the underlying pathophysiology of CAR-T related complications. A limitation in the clinic is to predict timelines of unwanted effects because of the ability of CAR-T cells to persist and expand in vivo. Another limitation in the future will be to determine if the detected side effects of CD19-targeting CAR-T cells to date may also apply to future CAR-T cell therapies targeting other antigens.
A major benefit of CD19-targeting CAR-T cells is the high efficacy in chemotherapy-refractory lymphoma and leukaemia. It is possible to cure about 30-40% of patients in this situation as compared with <10% long term survival using standard treatment approaches.
What are the emerging treatment pathways you’re most excited about in the CAR-T space?
There is a tendency to use CD19-targeting CAR-T cells in earlier lines of therapy. Currently, several randomized trials are investigating the value of CAR-T cell therapy in second-line therapy of large B-cell lymphoma.
On the other hand, emerging treatment pathways point towards an increasing use of CAR-T cell therapies targeting other antigens. In multiple myeloma, B-cell maturation antigen (BCMA)-targeting CAR-T cells are already in clinical use and in several other diseases, CAR-T cells are currently under investigation (for example, acute myeloid leukaemia, neuroblastoma, and inflammatory diseases).
According to the Global CAR-T Pipeline Insight Report 2020, there are approximately 250+ key companies developing therapies for CAR-T which makes it an exciting space to keep an eye on. The companies that have CAR-T drug candidates in the most advanced stage (phase III) include Janssen Research & Development, ViiV Healthcare, Sorrento Therapeutics, Celgene, Novartis, and Abbott.
How has COVID-19 impacted patients undergoing CAR-T therapy?
Data on COVID-19 in patients undergoing CAR-T cell therapy is sparse. However, it was recently reported that in a cohort of 77 patients with SARS/COV-2 infection receiving cellular therapy (autologous, allogeneic and CAR-T) had a favorable outcome.
Overall survival at one month after cellular therapy was >75%. This data suggests that COVID-19 infections in CAR-T cell recipients might be manageable. Nevertheless, robust data in a larger cohort is pending.
The study I refer to above can be found here: Favourable outcomes of COVID-19 in recipients of hematopoietic cell transplantation
What are the biggest remaining unmet needs for patients when it comes to CAR-T?
The biggest unmet need in CD19-targeting CAR-T cells is to increase the efficacy. Currently, far more than 50% of CAR-T cell treated patients will relapse and eventually die from the underlying disease.
A major unmet need regarding CAR-T outside the CD19-setting is on-target off-tumor toxicity. This occurs when CAR-T cells attack non-tumor cells expressing the target antigen. The difficulty is that there is a lack of suitable tumor neo-antigens and the majority antigens that are under investigation in CAR-T cell trials have a considerable expression in normal tissues. As a result, the use of CAR-T cells for the treatment of solid tumors may have an increased risk of severe toxicities.
What difficulties exist in referring patients for CAR-T therapies?
CAR-T cell therapy is relatively new. As such, networks and knowledge on CAR-T cells have to be established in the community. Currently, not all patients who need CAR-T cells end up being treated in CAR-T cell centers.
What are some of the barriers to widespread adoption of CAR-T and accessibility for patients?
A major barrier is the high price of this therapy leading to regulatory restrictions in most countries. Another major barrier is the individual manufacturing process with manufacturing failure in 10-30% of cases.
What do you see as the future potential and applications for CAR-T cell therapy?
Currently, numerous trials in further tumor entities are underway, making it likely that in the near future there will be a broader clinical use of CAR-T cells with different antigen specificities. In addition, inflammatory diseases are an interesting and innovative application for CAR-T cell therapy.
CAR-T is presently leading the charge in cancer treatment but are there any other emerging promising treatment modalities we should be aware of?
CAR-T cells are very promising. However, the field of cancer therapy is very innovative and rapidly developing. Of note, immunotherapies with checkpoint inhibitors, bispecific antibodies and antibody-drug-conjugates are very promising treatment options.